Shiv Nadar Institution of Eminence
On August 3, 2022, Shiv Nadar University, Delhi-NCR, became the youngest university to be recognized as an Institution of Eminence by the Ministry of Education, Government of India. From the undergraduate to the doctoral levels, we offer distinctive degree programs, foster cutting-edge research, and work for the betterment of society.
Explore our Schools
Research and learning at Shiv Nadar takes place across four Schools imbued with our philosophy of multidisciplinarity and interdisciplinarity.

School of
Management and
Entrepreneurship
Shiv Nadar IoE’s School of Management & Entrepreneurship (SME) believes in fostering the development of future-ready global leaders; adept at fueling innovative, pertinent, and implementable solutions for the challenges posed by the new digital economy of the future.
School of
Natural Sciences
The School of Natural Sciences provides students a global environment with innovative and engaging teaching methods. It offers a wide range of physical and biological science subjects across Chemistry, Life Sciences, Mathematics, and Physics at the undergraduate, postgraduate, and doctoral levels.

School of
Engineering
The School of Engineering guides graduates to the higher reaches of their profession at national and international levels.

Academy of
Continuing Education
The Academy of Continuing Education aims to facilitate best-in-class knowledge, practices, and skill development offerings to the growing ecosystem of lifetime learners and leaders, both within and outside the University.

School of
Humanities and Social Sciences
The School of Humanities and Social Sciences is an academically vibrant entity with departments that range from the purely academic to practice-oriented disciplines.
Find Your Program
The Shiv Nadar Impact
Erosion Resistance Bimodal Composite Coating Composition And Method Thereof
The University holds a patent for Erosion Resistance Bimodal Composite Coating Composition And Method Thereof by Harpreet Singh Grewal, Harpreet Singh Arora, Abhishek Babu
Achal Vinod
"The guidance I received from mentors at the University was unparalleled. During my course at the University, I got opportunities to explore my capabilities which helped me hone my skills and knowledge base."
pursuing M.Sc. Quantum Fields and Fundamental Forces at Imperial College London
Rakesh Munnanooru
I got an idea of a business model and how to go about it after I picked courses from the School of Management while studying for my B.Tech in the University. The case study discussions were of immense help. The multidisciplinary approach also helped me pick courses in economics which have helped me set up my business
Founder & CEO of WhistleDrive
Invention of Imidazole Derivatives
The University was awarded the patent for the invention of Imidazole derivatives, and Benzimidazole was used to degrade various toxic heavy metals and salts to a less toxic, stable, and insoluble form. The invention also provides the process of preparation of derivatives of imidazole and Benzimidazole based thiones and selones and a method of detoxification and degradation of various heavy metals, including mercury in particular organomercurials, lead, arsenic, cadmium, copper, and salts.
Recent Research
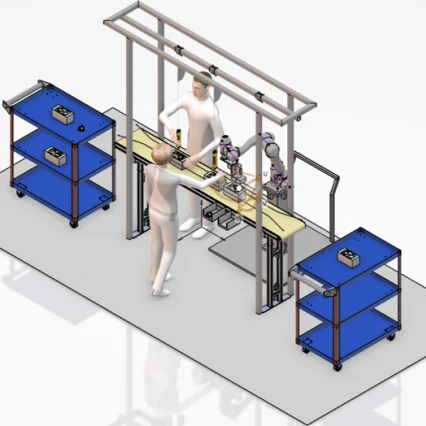
A Digital Twin Development for Collaborative Human-Robot Work Allocation in Assembly Line System
A digital twin is a virtual replica of a physical system that is used to simulate, analyse, and optimize its real-world counterpart. The project involves creating a digital model of an assembly...
Applications of structured random matrix models to some problems in classical and quantum information theories. (DST-SERB CRG/2022/001751)
In today’s world, it is unimaginable to survive without information exchange. The underlying mathematical framework, the information theory, serves as the basis of all communications, networking,...
Our Campus

The Best Part
Living on Campus
Biodiversity parks, culture labs, artist studios, a sports complex, and over 50 student bodies where like-minded learners come together and collaborate.














