Aging and Metabolism
Aging leads to organismal and cellular dysfunction and an increased risk for several diseases. Metabolic alterations such as deregulated nutrient signaling, mitochondrial dysfunction, and perpetual inflammatory responses are associated with age-related pathologies. Meanwhile, lifespan-enhancing interventions such as dietary restriction and modulation of nutrient-sensing pathways improve metabolism and increase health span. Research in Aging and metabolism is being conducted by investigators studying metabolic and chronic diseases influenced by lifestyle, aging, and epigenetic factors. These groups utilize the following cutting-edge methodologies to address different questions:
- Multi-omics approaches (spatial transcriptomics, small RNAseq, ATAC-seq, ChIP-seq, cut & run, proteomics, metabolomics, lipidomics)
- In vitro to animal models
- Behavioral and Survivability/lifespan assays under different contexts
- Immunofluorescence, Tissue imaging, live imaging, and Histochemistry
- Systems biology, interventional trial analyses, and AI-based Neuroinformatics
Central Facilities and Centers being utilized for the different research programs in the Aging and Metabolism theme: Common Instrumentation Facility (A, B, and R block), Center for Integrative and Translational Research (CITRes), Center for Excellence in Epigenetics (CoEE), Fly Facility, Core Imaging Facility, and Bioinformatics Facility.

Geetanjali Chawla, Ph.D.
Assistant Professor
Department of Life Sciences
Key focus areas:
- Post-transcriptional regulators of aging and dietary restriction.
- Dietary interventions that enhance healthspan and delay age-related diseases.

Colin Jamora, Ph. D.
Senior Professor
Head of Department of Life Sciences
Key focus areas:
- Regulation of fibroblast activity vis a vis ECM secretion and inflammation.
- Function and impact of senescent cells on skin homeostasis.

Sanjeev Galande, Ph. D.
Senior Professor and Dean
Department of Life Sciences
Key focus areas:
- Epigenetic aging of the brain.
- Impact of dietary changes on the brain.

Koyeli Mapa, Ph. D
Associate Professor
Department of Life Sciences
Key focus areas:
- Protein Homeostasis and Stress Response pathways.
- Integrated Stress response pathway, autophagy and mitophagy, molecular chaperones.

Prasun K. Roy, Ph. D.
Distinguished Professor, Department of Life Sciences
Head, Shiv Nadar IoE - Dassault Systemes Centre of Excellence
Key focus areas:
- Brain reorganization during lifespan.
- Systems biology analysis of neural growth and senescence.
- Cerebral white matter and grey matter adaptation.
- Signalling pathways, targets and drug identification relevant to aging.
Principal Investigator: Dr. Geetanjali Chawla, Associate Professor, Department of Life Sciences
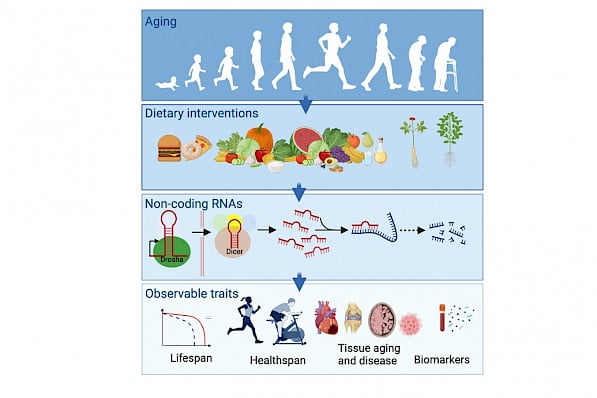
Aging is the most significant risk factor for several chronic diseases, including Alzheimer's disease, Type 2 Diabetes mellitus, and cardiovascular disease. Increasing evidence has implicated dietary restriction (DR), i.e., reduced nutrient intake that does not incur malnutrition, as a simple means of counteracting age-related disease. This nutritional intervention increases lifespan in diverse species, indicating that the molecular mechanisms that underlie DR are evolutionarily conserved. MicroRNAs (miRNAs) are a class of post-transcriptional regulators recognized as key players in aging and late-onset diseases. We employ genetic, molecular, metabolomic, and proteomic strategies to test whether RNA-mediated mechanisms operating downstream of DR can promote a healthy lifespan and ameliorate symptoms associated with age-related diseases. Auxiliary RNA-binding proteins (RBPs) function as critical components of signaling pathways that regulate lifespan by regulating microRNA biogenesis. Our work has also highlighted the importance of cis-acting elements such as terminal loops and stem-base in the differential expression of clustered miRNAs. As we advance, we are characterizing the functional role of RBPs in aging and age-related diseases.
Natural medicinal plants are a potential source of dietary interventions for the prevention and /or treatment of late-onset diseases. Though several medicinal plants have been used in healthcare for decades, the molecular mechanisms underlying their beneficial effects are poorly understood. We are utilizing omic-based and genetic approaches to uncover the molecular mechanisms underlying the beneficial effects of natural dietary interventions.
Collaborators:
Prof. Pankaj Kapahi (The Buck Institute for Research in Aging, Novato, CA, USA).
Dr. Matteo Micucci (Department of Biomolecular Sciences, University of Urbino “Carlo Bo”, 61029 Urbino, Italy).
Publications:
Wilson, K.A., Bar, S., Dammer, E.B. et al. OXR1 maintains the retromer to delay brain aging under dietary restriction. Nat Commun 15, 467 (2024). https://doi.org/10.1038/s41467-023-44343-3
Pandey M, Bansal S, Chawla G. Evaluation of lifespan promoting effects of biofortified wheat in Drosophila melanogaster. Exp Gerontol. 2022 Apr;160:111697. doi: 10.1016/j.exger.2022.111697. Epub 2022 Jan 10. PMID: 35016996; PMCID: PMC7613042.
Pandey M, Bansal S, Bar S, Yadav AK, Sokol NS, Tennessen JM, Kapahi P, Chawla G. miR-125-chinmo pathway regulates dietary restriction-dependent enhancement of lifespan in Drosophila. Elife. 2021 Jun 8;10:e62621. doi: 10.7554/eLife.62621. PMID: 34100717; PMCID: PMC8233039.
Link for complete list of publications: https://scholar.google.com/citations?user=k08GGgcAAAAJ&hl=en
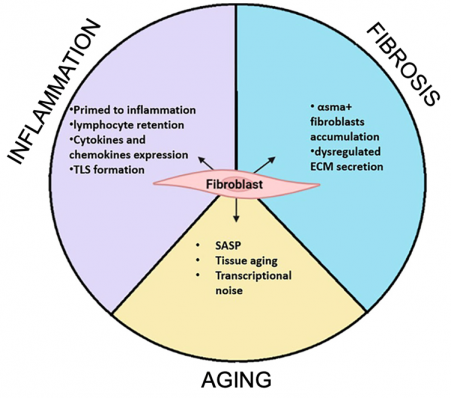
Image Credit: Gauthier V, Kyriazi M, Nefla M, Pucino V, Raza K, Buckley CD, Alsaleh G. Front Immunol. 2023 Feb 28;14:1137659. doi: 10.3389/fimmu.2023.1137659. PMID: 36926329; PMCID: PMC10011104.
Principal Investigator: Prof. Colin Jamora, Senior Professor and HoD, Department of Life Sciences
Fibroblasts represent a highly heterogeneous population of cells with distinct functional roles in tissue development, maintenance, repair, and pathology. This heterogeneity is particularly evident in the dermal compartment of the skin, where fibroblasts influence a broad range of physiological processes, including hair follicle cycling, epidermal regeneration, and wound healing. Notably, fibroblasts are integral to the aging process, contributing to the chronic, low-grade inflammatory state termed "inflammaging." Additionally, they play a central role in tissue fibrosis, a hallmark of age-related tissue dysfunction. Our research focuses on unraveling the molecular mechanisms driving the phenotypic shifts in fibroblast subpopulations that make them more inflammatory, pro-fibrotic, and prone to senescence. By elucidating these pathways, we aim to identify therapeutic targets that enable precise modulation of fibroblast activity to mitigate their contributions to aging and associated pathologies.
Collaborators:
Unilever: Microbiome in skin homeostasis.
Vivek Malhotra, PhD (Centre for Genomic Regulation, Barcelona): regulation of collagen content in the skin.
Publications:
Kataria, S., Rana, I., Badarinath, K., Zaarour, R. F., Kansagara, G., Ahmed, S. et al. (2024) Mindin/spondin-2 regulates fibroblast subpopulations through distinct Src family kinases during fibrogenesis JCI Insight 10.1172/jci.insight.173071
Rana, I., Kataria, S., Tan, T. L., Hajam, E. Y., Kashyap, D. K., Saha, D. et al. (2023) Mindin (SPON2) Is Essential for Cutaneous Fibrogenesis in a Mouse Model of Systemic Sclerosis J Invest Dermatol 143, 699-710 e610 10.1016/j.jid.2022.10.011
Pincha, N., Hajam, E. Y., Badarinath, K., Batta, S. P. R., Masudi, T., Dey, R. et al. (2018) PAI1 mediates fibroblast-mast cell interactions in skin fibrosis J Clin Invest 128, 1807-1819 10.1172/JCI99088
The link to the lab website where the entire list of publications can be found: https://jamoralab.weebly.com
Principal Investigator: Prof. Sanjeev Galande, Professor, Department of Life Sciences and Dean, School of Natural Sciences
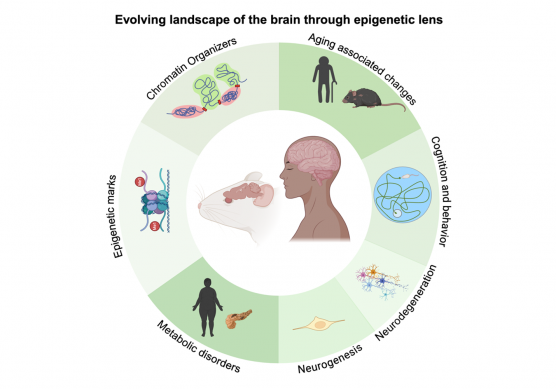
Our research focuses on metabolic changes in the nervous system during aging and how dietary modifications influence brain function and the aging process. Aging and metabolic disorders are deeply interconnected, and we aim to investigate these relationships in mammalian brain development. We study brain aging through two key hallmarks: epigenetic alterations and genomic instability. Our research explores how neural plasticity evolves with age and how epigenetic modifications regulate adult neurogenesis. Since neurogenesis declines significantly with age, we aim to understand the role of chromatin organizers in aging neural stem cells within neurogenic niches.
We examine how different diets influence the brain’s transcriptome, leaving lasting epigenetic marks. These age-associated changes are particularly pronounced during adolescence. Using rodent models, we investigate how dietary exposure affects brain function, focusing on inflammation in the gut-brain axis.
Collaborators:
Dr Nixon Abraham (Indian Institute of Science Education and Research, Pune).
Publications:
O'Rourke MB, Januszewski AS, Sullivan DR, Lengyel I, Stewart AJ, Arya S, Ma RC, Galande S, Hardikar AA, Joglekar MV, Keech AC, Jenkins AJ, Molloy MP. Optimised plasma sample preparation and LC-MS analysis to support large-scale proteomic analysis of clinical trial specimens: Application to the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial. Proteomics Clin Appl. 2023 May;17(3):e2200106. doi: 10.1002/prca.202200106. Epub 2023 Mar 22. PMID: 36891577; PMCID: PMC10909541.
Khare SP, Madhok A, Patta I, Sukla KK, Wagh VV, Kunte PS, Raut D, Bhat D, Kumaran K, Fall C, Tatu U, Chandak GR, Yajnik CS, Galande S. Differential expression of genes influencing mitotic processes in cord blood mononuclear cells after a pre-conceptional micronutrient-based randomised controlled trial: Pune Rural Intervention in Young Adolescents (PRIYA). J Dev Orig Health Dis. 2023 Jun;14(3):437-448. doi: 10.1017/S204017442200068X. Epub 2023 Jan 12. PMID: 36632790.
Pandit P, Galande S, Iris F. Maternal malnutrition and anaemia in India: dysregulations leading to the 'thin-fat' phenotype in newborns. J Nutr Sci. 2021 Oct 11;10:e91. doi: 10.1017/jns.2021.83. PMID: 34733503; PMCID: PMC8532069.
The link to the lab website where the entire list of publications can be found: http://www.sglabepigenetics.com/
Principal Investigator: Dr. Koyeli Mapa, Associate Professor, Department of Life Sciences
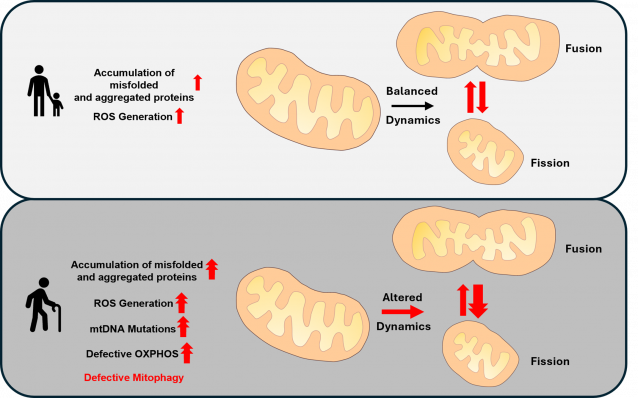
Our group is interested in elucidating mitochondrial protein homeostasis during different environmental stresses and aging. As individuals age, their cells progressively diminish in their capacity to manage stress. Mitochondria—often referred to as the powerhouses of the cell—play a central role in this age-associated deterioration stress managing capacity. With aging, there is an increased accumulation of misfolded and damaged proteins within various sub-compartments of eukaryotic cells including mitochondria, which activates stress response pathways aimed at maintaining cellular homeostasis. In the case of mitochondria, in younger cells, these mechanisms operate effectively, removing defective proteins and ensuring optimal energy production. However, as one ages these protective systems become less effective, resulting in chronic stress, reduced energy production, and heightened oxidative damage. Additionally, metabolic imbalances further burden the mitochondria, accelerating the process of cellular aging. This decline in mitochondrial quality control significantly contributes to the onset of age-related diseases. By gaining insights into how cells cope with mitochondrial stress, we may uncover strategies to decelerate the aging process and enhance overall quality of life and healthy aging.
Collaborators:
Dr. Nidhi Malhotra, Department of Chemistry, SNIoE
Dr. Animesh Samanta, Department of Chemistry, SNIoE
Dr. Rajan Vyas, Department of Life Science, SNIoE
Dr. Swasti Raychaudhuri, CSIR-CCMB, Hyderabad
Dr. Kausik Chakraborty, CSIR-IGIB, Delhi
Dr. Nikhil Gandasi, IISc Bangalore
Dr. Chinna P Swamy at NIT Calicut
Publications:
Narayana Rao KB, Pandey P, Sarkar R, Ghosh A, Mansuri S, Ali M, Majumder P, Ranjith Kumar K, Ray A, Raychaudhuri S, Mapa K. Stress Responses Elicited by Misfolded Proteins Targeted to Mitochondria. J Mol Biol. 2022 Jun 30;434(12):167618. doi: 10.1016/j.jmb.2022.167618. Epub 2022 Apr 30. PMID: 35500842.
Sarkar R, Rao KBN, Jha MP, Mapa K. Endoplasmic reticulum-unfolded protein response pathway modulates the cellular response to mitochondrial proteotoxic stress. Cell Stress Chaperones. 2022 May;27(3):241-256. doi: 10.1007/s12192-022-01264-2. Epub 2022 Mar 16. PMID: 35294718; PMCID: PMC9106787.
Ghosh A, Gangadharan A, Verma M, Das S, Matai L, Dash DP, Dash D*, Mapa K*, Chakraborty K*. Cellular responses to proteostasis perturbations reveal non-optimal feedback in chaperone networks. Cell Mol Life Sci. 2019 Apr;76(8):1605-1621. doi: 10.1007/s00018-019-03013-8. Epub 2019 Jan 25. PMID: 30683983; PMCID: PMC11105298.
Link for complete list of publications:https://scholar.google.co.in/citations?user=S1v0-okAAAAJ&hl=en
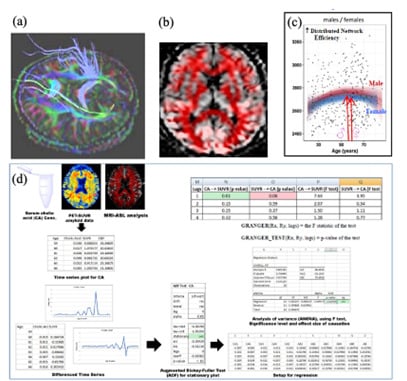
Fig. 1. (a) Non-invasive mapping of white matter tracts in mid-aged brain. (b) Noninvasive mapping of vascular flow field in elderly brain. (c) Maximum circuit networking efficiency of both male and female human brain occurs around 6th-7th decade of age. (d) Causation Analysis methodology and platform that we developed for finding the intensity and direction of causative linkages in the pathophysiological networks of different tissues as human aging occurs.
Principal Investigator: Prof. Prasun K. Roy, Distinguished Professor, Department of Life Sciences; Head, Shiv Nadar IoE - Dassault Systemes Centre of Excellence.
Using lifespan-based non-invasive investigation of brain structure and function, we study how behavioural functions are well maintained in advanced age, even though degeneration and brain shrinkage exist. Therefore, we demarcate the causative factors for behavioural compensation and resilience. We also extend our lifespan study to include the foetal span and map how the prenatal and postnatal brain develops, thereby initiating the longevity trajectory across growth and aging. Moreover, innovative AI-based neuroinformatics techniques are also formulated by us to analyse and draw mechanistic insights from large-scale human studies. Furthermore, in cerebral hazards (like brain injury or cognitive decline), we utilize systems biology analysis, imaging methodology and interventional trial analyses, to delineate out the dysfunctional pathways and home onto novel therapeutic candidates. The graphics below (Fig. 1) illustrate some of our work.
Collaborators:
Dr. L Raghavendran, Jawaharlal Nehru University
Dr. Willem Otte, Utrecht University Medical Centre
Dr. Ashish Verma, Banaras Hindu University Institute of Medical Sciences
Dr. Chandrasekhar Sakode, Indian Institute of Information Technology Nagpur
Dr. Partha Mitra, Cold Spring Harbor Laboratory
Dr. Ashutosh Singh, Shiv Nadar University
Dr. Prasun Dutta, Indian Institute of Technology (BHU)
Publications:
Bhattacharjee A, Purohit P, Roy PK. Neuroimaging-based drug discovery for amyloid clearance therapy in Alzheimer's disease using validated causation analysis. Psychiatry Research: Neuroimaging, 345: 11890, 1-14, 2024.
Bhattacharjee A, Roy PK. Conjoint hepatobiliary-enterohepatic cycles for amyloid excretion and enhancing its drug-induced clearance: A systems biology approach to Alzheimer’s disease. Journal of Biomolecular Structure and Dynamics. 12, 1-24, 2023.
Bhattacharjee A, Roy PK. MRI tractographic validation of drug-enhanced hepatic clearance of amyloid-beta and the therapeutic potential for Alzheimer’s Disease: A pilot study. Brain Disorders, 13:100112, 1-9, 2024.
Link for entire list of publications: https://rb.gy/3gwszz