Microbes and Immunity
Research in this domain explores the complex interactions between microorganisms and the immune system, focusing on their roles in health and disease. Imbalances in microbial communities can lead to disorders and recent studies aim to leverage microbial metabolites and immune modulatory strategies to restore balance and develop therapies for a range of conditions. This multidisciplinary approach offers promising solutions for enhancing immune response and improving health outcomes.
Colin Jamora
The skin is the body’s largest and most external physical barrier, offering protection against harmful microorganisms, toxins, allergens, and extreme environmental factors. It also functions as an immunologically active organ, containing a range of immune effector molecules and resident cells from both the innate and adaptive immune systems, collectively forming a robust immunological barrier.
Despite being constantly exposed to diverse microorganisms, the skin has an extraordinary ability to differentiate between harmless commensal microbes and harmful pathogens. Commensal microbes naturally inhabit the skin without causing harm and often provide benefits, such as preventing the colonization of pathogenic bacteria. Conversely, pathogens take advantage of vulnerabilities like breaks in the skin barrier to invade and damage host tissues. The relationship between the skin's immune system and its microbiota is dynamic: the immune system can regulate microbial populations, while the microbiota plays a role in educating and shaping immune responses. When this balance is disrupted—a condition known as skin dysbiosis—it can lead to the onset of skin conditions such as acne, psoriasis, and atopic dermatitis.
Ultimately, the skin’s role as an immunological barrier is highly complex, relying on the interplay between its physical structure, immune mechanisms, and microbial ecosystem. This intricate coordination is essential for maintaining skin health and defending against harmful pathogens.
Highlights:
- Mechanisms by which the skin microbiota affect aging, immunity, and disease
- Regulation of antimicrobial peptides in the skin to combat infection and promote skin homeostasis
- Intercellular cross-talk between immune cells and other cutaneous cells
- Novel functions of the innate and adaptive immune systems in tissue repair and regeneration
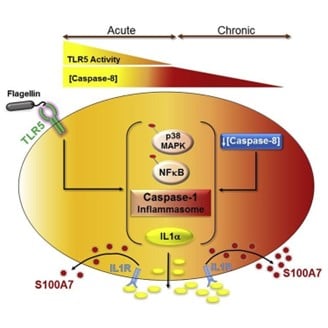
As part of its innate immune system, the skin produces and stores a large reservoir of diverse antimicrobial peptides (AMPs) with a wide spectrum of anti-viral and anti-bacterial activities. In addition these AMPs can regulate the adaptive immune system, thereby eliciting a comprehensive response to microbial infections. In collaboration with Unilever, the Jamora lab has been investigating the molecular mechanisms regulating the expression and secretion of AMPs from the skin in order to boost defense against potential infections. (Figure from DOI: 10.1016/j.celrep.2019.10.090)
Webpage link: https://jamoralab.weebly.com
Active Collaborations:
- Unilever
- Koshkey Pvt. Ltd.
Relevant facilities:
- Centre for Integrative and Translational Research (CITRes)
- Imaging facility
- Sequencing facility
Richa Priyadarshini
Human microbiome has gained importance in the past decade and its role in health and diseases is well established. Oral microbiome is a highly complex microbial community residing in the oral cavity. More than 700 bacterial species are found in oral communities and is considered the second largest microbial community in the human body. Dysbiosis of these microbial communities leads to periodontal diseases such as periodontitis and dental caries. Lactobacilli are a part of the normal microbiota in the oral cavity and gastrointestinal tract. Lactobacillus species have been studied for a long time for their potential use as probiotics. Probiotic lactobacilli interact with other bacteria in the biofilm and hamper growth of pathogens through production of hydrogen peroxide, bacteriocins, and organic acids. While organic acids lower the pH, which promotes the growth of acid-tolerating bacteria, the production of bacteriocins may inhibit the growth of other pathogenic species. Apart from these lactobacillus species can also produce small organic metabolites which modulate the growth of bacterial biofilms. Our research involves isolation and characterization of small organic metabolites/peptides from Lactobacillus with anti-microbial properties.
Highlights:
- Creating a library of small organic metabolites/peptides produced from Lactobacillus species
- Evaluation of antimicrobial properties of small effector molecules/peptides
- Use of small metabolites/peptides to modulate biofilm mediated diseases
- Understanding the underlying mechanism of antimicrobial effect
Funding:
SERB-YSS, ICMR, L’Oréal
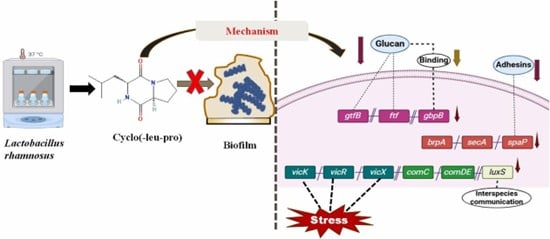
Niranjan, R., Patil, S., Dubey, A., Lochab, B., & Priyadarshini, R. (2024). Biofilm, 8, 100237. https://doi.org/10.1016/j.bioflm.2024.100237
Rajan Vyas
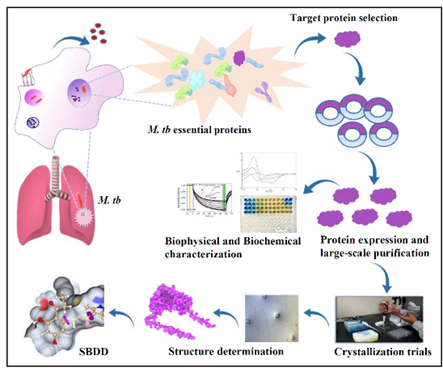
Tuberculosis (TB) is a contagious bacterial infection caused by Mycobacterium tuberculosis (M. tb), primarily affecting the lungs, though it can spread to other parts of the body. TB is treatable with antibiotics, but if left untreated, it can be fatal. The TB treatment is lengthy and typically involves a combination of four first-line drugs taken over six months. The patients must complete the entire course of treatment to eradicate the disease and avoid the origin of multiple drug-resistant (MDR) and extremely drug-resistant (XDR) resistance strains, which require treatment involving second-line drugs for up to 18-24 months. Thus, there is a requirement to find novel drug candidates along with their inhibitors to help eradicate the MDR and XDR strains and reduce the length of the therapy. The essential M. tb proteins involved in its survival, pathogenesis, and virulence are potential drug targets and can be explored to discover new therapeutic drugs. Our research mainly focuses on biochemical and biophysical characterization of the essential proteins with the ultimate aim of identifying potential inhibitors using structure-based drug design that pave the way for novel drug development.
Highlights:
- Structural and functional characterization of clinically important proteins or essential metabolic pathway proteins using macromolecular protein crystallography.
- Deducing mechanistic insights and identifying potential inhibitors as a structure-based drug-designing approach.
Relevant facilities:
- Protein expression and purification facility
- Protein analysis facility – Circular dichroism (CD) spectrophotometer, Microscale thermophoresis (MST)
Jugal Das
Cancer and autoimmune diseases represent extremes of immune dysfunction: cancer involves an immunosuppressive environment that allows tumor growth, while autoimmune diseases are driven by an overactive immune system causing chronic inflammation and tissue damage. Despite these differences, both conditions share regulatory pathways that offer potential therapeutic targets. Immunotherapy aims to restore immune balance by enhancing regulation in autoimmune diseases and overcoming suppression in cancer, such as targeting T cell populations to either control inflammation or reactivate immune surveillance against tumors.
Advances in cancer immunotherapy have centered around approaches such as immune checkpoint inhibition, T cell expansion, and engineered T cell platforms, including chimeric antigen receptor (CAR) T cells. Targeting major immune checkpoints such as PD-1/PD-L1 axis or CTLA-4 has shown success in revitalizing T cells for robust anti-tumor responses. Complementary strategies focus on enhancing T cell infiltration into tumors and prolonging their functional activity in the tumor microenvironment.
From the gut to the soil, microbes are silent partners in life’s grand design. An emerging aspect of research explores the use of microbes and their metabolites as immunomodulatory agents. This capability makes them valuable tools for both suppressing excessive inflammation in autoimmune disorders and enhancing immune activity in cancer.
Understanding the intricate interplay between immune activation and suppression provides the foundation for innovative therapeutic strategies. The focus of the lab is to integrate conventional and novel immunomodulatory approaches, to develop balanced therapies for cancer and autoimmune diseases aiming for translation.
Highlights:
- Cancer thrives in an immunosuppressive environment, while autoimmune diseases result from hyperactive immunity.
- Both share regulatory pathways, offering therapeutic targets.
- Cancer: Boost T cell activity to overcome suppression.
- Autoimmunity: Suppress excessive immune responses to restore balance.
- Checkpoint inhibitors reactivate T cells.
- CAR-T cells and adoptive cell therapy strategies to enhance T cell infiltration.
- Microbes and their metabolites suppress inflammation in autoimmunity and enhance immunity in cancer.
Lab Focus:
- Combining traditional and novel approaches to develop balanced therapies for cancer and autoimmune diseases.
- Developing microbe based immunotherapeutic strategies to balance immune responses to overcome autoimmune diseases and cancer.
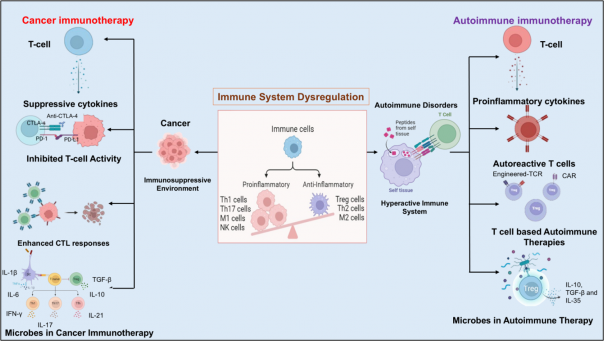
Immune dysregulation in cancer and autoimmune diseases stems from T-cell imbalances. In cancer, T cells are suppressed, hindering tumor targeting, while in autoimmunity, overactive T cells cause tissue damage. T-cell therapies aim to restore balance: checkpoint inhibitors activate T cells against tumors, whereas autoimmune treatments suppress harmful T-cell activity. Microbes can modulate both these processes.
Link to webpage: https://jk-impactlab.in/
Active Collaborations:
Jianxun (Jim) Song, Endowed Professor in Immunology, Texas A&M University, USA
Relevant Facilities:
- Center for Integrative and Translational Research (CiTRES)
- Flowcytometry Facility- BD FACS Aria 3 Cell sorter, Beckman Coulter CytoFLEX- 3 lasers, 13 colors analyzer
- Imaging Facility- Confocal and Fluorescence Microscopes